Risk of Congenital Malformations With Statin Therapy During the First Trimester of Pregnancy
ABSTRACT
Background: Statins are the most common and effective drug used to treat hyperlipidemia. Based on animal studies showing potential teratogenic effects at high doses, statins are contraindicated in pregnancy due to concern that they would disrupt cholesterol biosynthesis for the fetus. This review evaluates the current evidence of harm caused by statins when taken within the first trimester of pregnancy.
Methods: An exhaustive literature search was conducted in June 2015 using MEDLINE-Ovid, CINAHL, Evidence-Based Medicine Reviews Multifile, and Web of Sciences databases. Keywords searched included statin, pregnancy, and congenital malformation. The search was further narrowed down to include only English-language articles and human studies published within the last ten years. Articles within a ten-year period that evaluated the possible congenital malformations of statin drugs in first trimester women were included. The Grading of Recommendations Assessment, Development and Evaluation (GRADE) Working Group Guidelines was used to evaluate the quality of the remaining eligible articles.
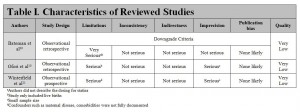
Results: Three articles met the inclusion criteria for this systematic review. One retrospective study looked at 886 996 completed pregnancies, 1152 of which took a statin during their first trimester, and found no significant teratogenic effects from first trimester statin therapy. The second retrospective cohort study with 288 pregnant women found no significant teratogenic effects from first trimester statin therapy. The prospective observational study included 498 women found no significant teratogenic effects from first trimester statin therapy. All studies were rated as having low quality of evidence based on GRADE guidelines.
Conclusion: Statins may not be as harmful during pregnancy as the FDA’s class X designation advises, but there is not enough statistical strength to change the current recommendation of the discontinuation of statins during pregnancy. Additional research to evaluate the long-term effects of in utero exposure to statins is needed.
Keywords: Statin, pregnancy, congenital malformation
(Click on image to enlarge.)
REVIEWED STUDIES:
Ofori B, Rey E, Berard A. Risk of congenital anomalies in pregnant users of statin drugs. Br J Clin Pharmacol 2007;64:496–509.
Bateman BT, Hernandez-Diaz S, Fischer MA, Seely EW, Ecker JL, Franklin JM. Statins and congenital malformations: cohort study 2015; 350 :h1035
Winterfeld U; Allignol A; Panchaud A; Rothuizen LE; Merlob P; Cuppers-Maarschalkerweerd B; Vial T; Stephens S; Clementi M; De Santis M; Pistelli A; Berlin M; Eleftheriou G; Mañáková E; Buclin T. Pregnancy outcome following maternal exposure to statins: a multicentre prospective study. BJOG: An International Journal of Obstetrics & Gynaecology. 2013;120(4):463-71
AUTHOR: Alexander Hoffman is currently completing his second year in the School of PA Studies at Pacific University, Oregon. He will graduate with an MS in Physician Assistant Studies in August, 2016.